This bond links two consecutive alpha-amino acids from C1 carbon number one of one alpha-amino acid and N2 nitrogen number two of another. The rRNA acts both to secure the mRNA and tRNA in the ribosome and as a catalyst to speed the formation of peptide bonds between amino acids.
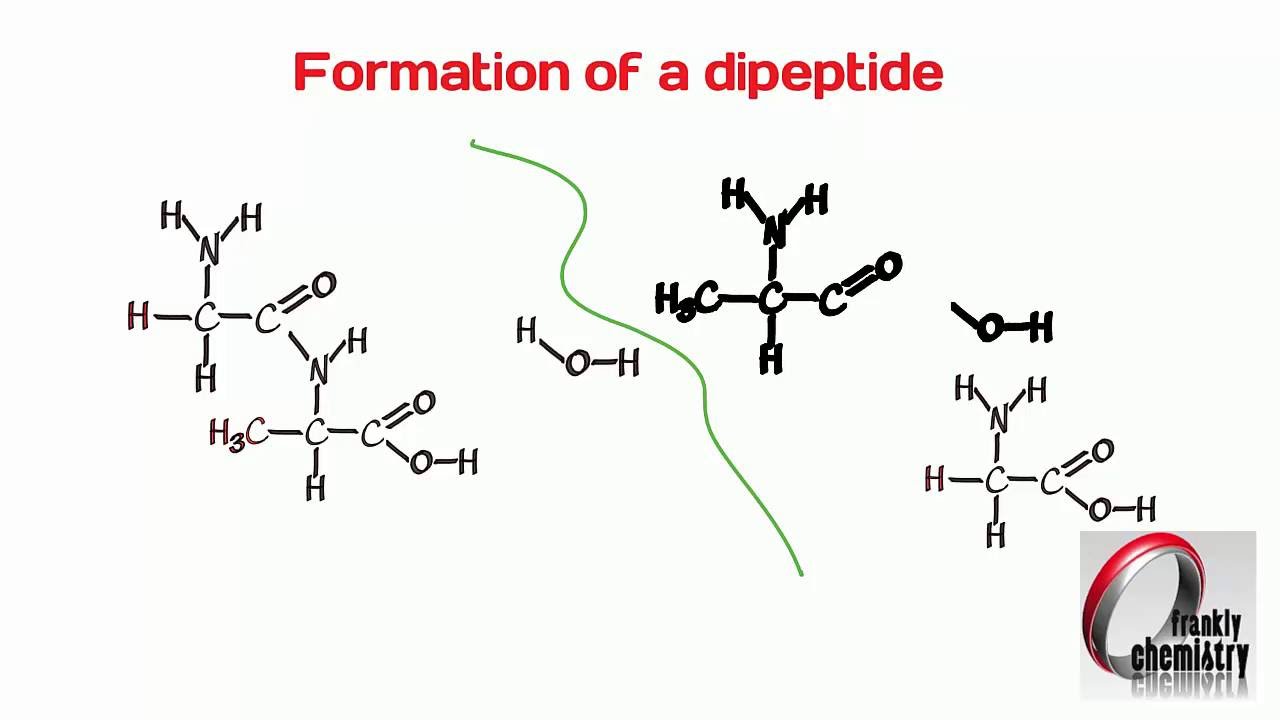
Amino Acids 4 Formation Of A Dipeptide Youtube
Draw the structure of eacha Val b.
. The bromine rebound reactivity is promoted by the conversion of a weaker FeBr bond in the ferric bromide species 6 to a stronger primary Csp 3 Br bond in 2a. The fast trapping of the carbon radical via bromine atom transfer renders the CC bond formation step irreversible and enables kinetic control of reaction stereochemistry. Dehydration synthesis is also called as condensation reaction because both are characterized by the condensation and formation of water from the large molecule.
The first organocopper compound the explosive copperI acetylide Cu 2 C 2. Rhodium-Catalyzed Transannulation of 45-Fused 1-Sulfonyl-123-triazoles with Nitriles. However breaking a peptide bond this way occurs at a much slower rate than the dehydration synthesis reaction.
They are reagents in organic chemistry. What amino acids do the following abbreviations stand for. Polysaccharides may differ according to the type of monosaccharide they possess and the way the subunits bond together.
Nucleotides form bonds between the pentose sugar and phosphate group to form long polynucleotide chains. Peptide bond formation. Beryozkina Daan Willocx Pavel S.
Visible Light-Promoted Amide Bond Formation via One-Pot Nitrone in Situ FormationRearrangement Cascade. Overview of the collagen triple helix. Interfere with aminoacyl translocation reactions prevent release of uncharged tRNA from donor site after peptide bond is formed Erytnromycin 31.
Organocopper compounds is the chemistry of organometallic compounds containing a carbon to copper chemical bond. The Selective Formation of 1-Sulfonyl-45-fused Imidazoles versus Secondary CH Bond Migration. Formation of tetrahydrofolic acid.
This linkage is found along a peptide or protein chain. Silaichev Santhini Pulikkal Veettil Wim Dehaen and. This fast reaction under mild conditions should be useful in synthesis.
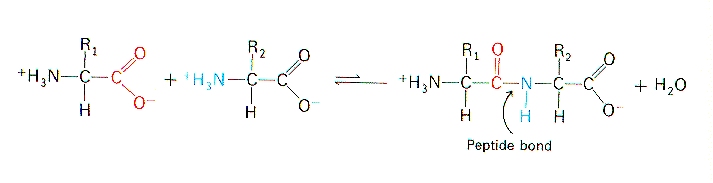
This process continues giving long polypeptide chain of aminoacids. Peptide formation occurs between the amino acids in the P site and the A site in the presence of the enzyme peptidyl transferase. In this kind of condensation two amino acids approach each other with the non-side chain C1 carboxylic acid moiety of one coming near the non-side chain N2 amino moiety of the otherOne loses a hydrogen and oxygen from its carboxyl group COOH and the other loses a.
The formation of a peptide bond requires energy in the form of adenosine triphosphate ATP. A novel amide bond formation strategy from simple thioacid and amine starting materials is mediated by unstable but very reactive S-nitrosothioacid intermediates. One class of substances called alkaloids found in many of these plants has been isolated and found to contain cyclic molecules with an amine functional groupThese amines are bases.
Bao-Gui Cai Shuai-Shuai Luo Lin Li Lei Li Jun Xuan and. Each subunit is a combination of proteins and RNA called ribosomal RNA rRNA. A peptide-based synthon for liquid.
A catalytic hydrogen transfer reaction using PdC HCONH 4 and the micelle-forming surfactant TPGS-750-M for hydrogenolysis of Cbz-protected amines and benzyl protected alcohols and hydrogenation of alkenes alkynes nitros nitriles halides and aldehydes of DNA-conjugated substrates is described. Bond between tw o D-alanyl residu es in the pentapeptide and become acy lated. A peptide bond is basically an amide-type of covalent chemical bond.
Amino acids are joined by peptide bond with loss of one water molecules between two amino acids. This rRNA is exists in various strands of different length and is surrounded by the many proteins that create a ribosome. Huimin Xue Jinbo Fei Aoli Wu Xia Xu and.
A First high-resolution crystal structure of a collagen triple helix formed from ProHypGly 4 ProHypAlaProHypGly 5 Protein Data Bank PDB entry 1cag b View down the axis of a ProProGly 10 triple helix PDB entry 1k6f with the three strands depicted in space-filling ball-and-stick and ribbon representation. The second tRNA now carries a dipeptide and the first tRNA is without an amino acid. When two amino acids form a dipeptide through a peptide bond it is a type of condensation reaction.
Although some attention has been given to the activation of acyl imidazoles with Brønsted acids these species can be orders of magnitude less reactive than the N-alkylated analogues as was noted by Jencks and Lapshin in their kinetic studies. The dipeptide D-alanyl-D-alanine is synthesiz ed from. Acyl imidazoliums have been long recognized as highly reactive acyl transfer agents in the context of amide bond formation.
Interfere with formation of initiation complexes for peptide chain synthesis 2. This is because amino acid residues have an extra double bond. The aminoacid present in t-RNA of P-site ie Fmet is transferred to t-RNA of A-site forming peptide bond.
With further additions resulting in the formation of a polypeptide chain. The peptidyl transferase reaction transfers the amino acid from the P site onto the amino acyl-tRNA in the A site. During the formation of this bond there is a release of water H 2 O.
Organocopper chemistry is the study of organocopper compounds describing their physical properties synthesis and reactions. The dipeptide on P-site is transferred to A-site forming tripeptide. Since ancient times plants have been used for medicinal purposes.
Gas-Induced Phase Transition of Dipeptide Supramolecular Assembly. Formation of a Dipeptide. It is possible to reverse this reaction by adding water.
The methodology is fully compatible with DNA. Liquidliquid phase separation plays an important role in creating cellular compartments and protocells but designing small-molecule models remains difficult. They can react with H 3 O in a dilute acid to form an ammonium salt and this property is used to extract.
Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars production of proteins from amino acids conversion of fatty acids to complex fats and the.
Peptide Bond Formation Biochemistry Flashcards Draw It To Know It

Peptide Bond Definition Structure Mechanism And Examples
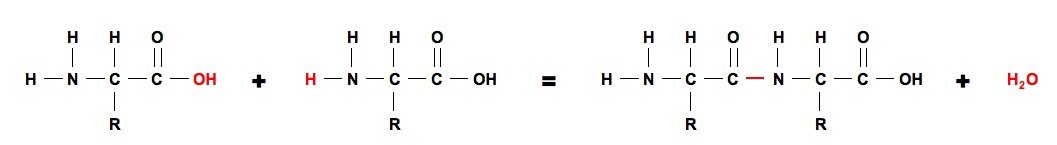
0 Comments